00063
Down-puckering of Hyp destabilizes the triple-helical structure
Department of Macromolecular Science, Graduate School of Science, Osaka University* Department of Macromolecular Science, Graduate School of Science, Osaka University, Japan** Instrumentation Analysis Center, Tokyo University of Agriculture & Technology, Japan*** Faculty of Engineering, Kyushu Institute of Technology, Japan****
○Chizuru Hongo* Kenji Okuyama** Keiichi Noguchi*** Shutoku Ebisusaki**** Norikazu Nishino****
Collagen is a major fibrous protein in animal body. Its amino acid sequence is usually assumed to be (Gly-X-Y)n, where X and Y positions are frequently occupied by a proline (Pro) and a 4(R)-hydroxyproline (Hyp), respectively. These features enable collagen molecules to form a stable triple-helical structure. Especially, Hyp in the Y position facilitates this helical formation. However, it was also known that Hyp in the X position destabilizes the helix.
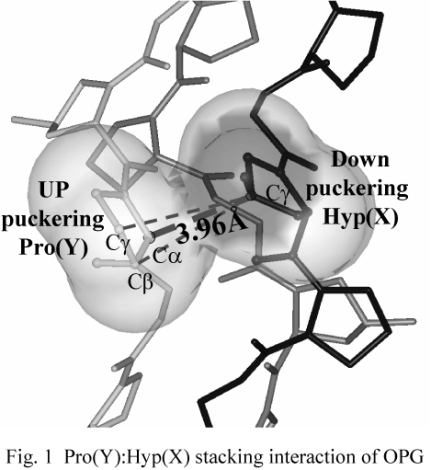
In this study, the puckering of Hyp in the X position was investigated by using a host-guest peptide, (Pro-Pro-Gly)4-Hyp-Pro-Gly-(Pro-Pro-Gly)4 (hereafter OPG). X-ray data collections were performed at BL40B2 of the SPring-8. The structure was analyzed at 1.36 Å resolution. In this structure, Hyp in the X position adopted a down-puckering in spite of energetically stable up-puckering and formed van der Waals stacking interactions with Pro in the Y positions of the adjacent strand (Fig. 1). These interactions are available only when imino acids in the X and Y positions adopt down- and up-puckerings, respectively. Although there is some compensation by the Pro(X):Hyp(X) interaction, the major factor to destabilize the OPG helix seems to be the unfavorable down-puckering of Hyp.