00081
Crystal Engineering of Microporous Chiral Coordination Polymers based on L-tartrate Ligands
Chemistry, HKUST
○Ian D. WILLIAMS
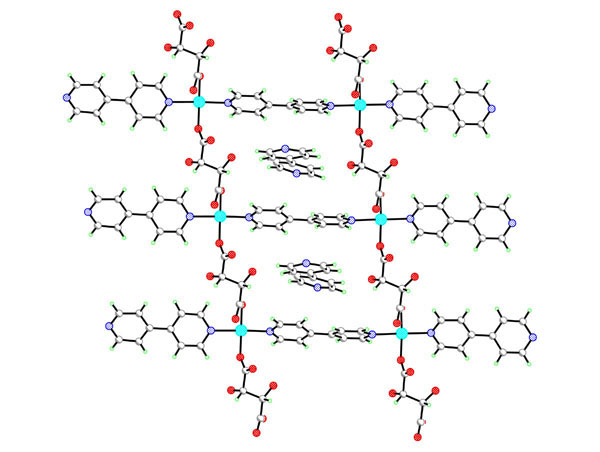
L-tartrate anions can survive hydrothermal synthesis up to 160oC enabling formation of the chiral porous lanthanide tartrates [Ln2(L-TAR)3(H2O)2]3H2O. [1] This prompted us to look at the hydrothermal crystal chemistry of a variety of metal tartrate systems, including M2+, M3+[2] and vanadium M4+ ions. In general dimensional and topological control of the crystalline phases formed is exerted through use of elevated temperatures to reduce ancillary ligation. The use of co-ligands can also be employed to engineer the channel size and structure for 3D network polymers, thus the mixed [Ln2(L-TAR)2(SUC)(H2O)2]5.5H2O with larger 1D channels can be formed by adding succinate and topomeric open frameworks of [Cu(L-TAR)(bipy)]3H2O or [Cu(L-TAR)(bipy)][bipy] (see fig) can be formed on addition of 4,4'-bipyridine. Stabilities up to 250oC have been found for these microporous frameworks by in situ variable temperature pXRD.
1. S. Thushari, J.A-K. Cha, H. H-Y. Sung, S. S-Y. Chui, I. D. Williams, Chem. Commun., 2005, 5515.
2. A. S-F. Au-Yeung, J.A-K. Cha, H. H-Y. Sung, S. S-Y. Chui, I. D. Williams, Inorg. Chem. Commun.,
2006, 9, 507. The RGC is thanked for financial support of this work under grant 6084/02P.