Chemistry, HKUST* Department of Biochemistry, HKUST, Hong Kong**
○Fanny L-Y Shek* Wan-Yee Wong* Herman H-Y. Sung* Hong Xue** Ian D. Williams*
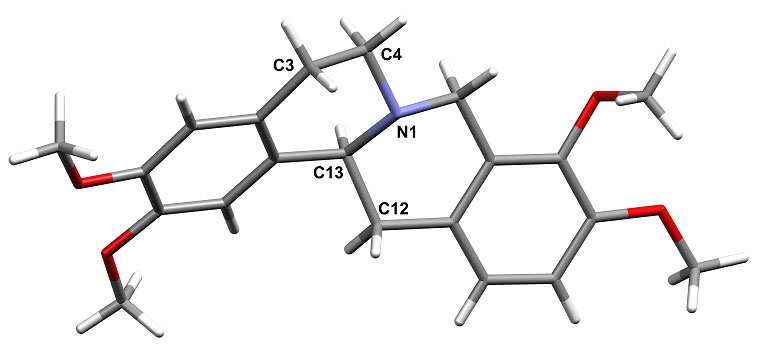
Derived from various Traditional Chinese Medicinal herbs, Tetrahydropalmatine THP C21H25NO4 (below) is a dopamine receptor antagonist used as anti-anxiolytic agent (H. Xue, HKUST 2004) It is a chiral molecule and can occur as both rac and active (-)-form. We have looked at forming salts of this through hydrothermal crystallization, for enhanced stability, solubility and possible chiral resolution of the isomer forms. A wide range of salts have been prepared using various organic acids. Chiral resolution appers possble using D-tartaric acid. Examination of the forms from rac-THP with various benzoic acids shows an interesting solid state pKa phenomenon. For acidic benzoates proton transfer to the nitrogen occurs and a salt is formed, but less acidic ones form a neutral molecular adduct with N---HO H-bond. This switch is found for the 4-Cl and 4-Br derivatives at pKa ca 4.0. Finally hydrothermal reaction allows hydrolytic stability of drug molecules to be assessed. Long time hydrothermal reaction (6d, 110oC) of either rac- or (-)-THP eventually leads to the new oxidation product Dihydropalmatine (DHP) C21H23NO4 in good yield and purity. The RGC is thanked for financial support of this work (grant HKUST 6084/02P).
