00084
Dimensional and Structural Control of Magnesium Tartrate Coordination Polymers
Chemistry, HKUST
○Pokka K-C. Pang John A-K Cha Andy L-F Leung Herman H-Y Sung Alvin W-H Siu Yu-fong Yen Ian D Williams
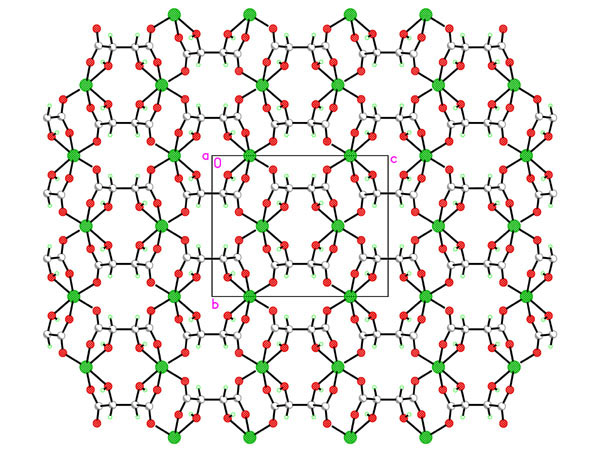
Hydrothermal synthesis of the chiral porous lanthanide tartrates [Ln2(L-TAR)3(H2O)2]3H2O [1] as well as indium tartrates [2] prompted us to look at the group 2 analogues. Reaction of magnesium acetate with L-tartaric acid, rac D/L-tartaric acid or meso-tartaric acid under a variety of conditions was studied in-depth and a variety of phase types [Mg(TAR)(H2O)x]H2Oy with variable hydration and dimensionality discovered. For L-tartrate at lower temperatures the 2D sheet polymer [Mg(L-TAR)(H2O)2] 1 is formed, whist temperatures to 180oC yield [Mg(L-TAR)]H2O 2, a tetragonal phase with small aquated micropores. Compound 1 smoothly and irreversibly converts to an anhydrous phase [Mg(L-TAR)] 3 (see fig) in a single crystal-single crystal transformation. This new phase type 3, has a different topology from isomeric 2. Despite the phase diversity all tartrate binding to Mg2+ is dominated by 1,2-chelation of a carboxylate oxygen and the b-hydroxy group.
1. S. Thushari, J.A-K. Cha, H. H-Y. Sung, S. S-Y. Chui, I. D. Williams, Chem. Commun., 2005, 5515.
2. A. S-F. Au-Yeung, J.A-K. Cha, H. H-Y. Sung, S. S-Y. Chui, I. D. Williams, Inorg. Chem. Commun.,
2006, 9, 507. The RGC is thanked for financial support of this work under grant 6084/02P.