00181
Photoinduced Reversible Microfibril Formation on a Photochromic Diarylethene Microcrystalline Surface
Yokohama Laboratory, Mitsubishi Chemical Group Science And Technology Research Center, Inc.* Department of Materials Chemistry, Faculty of Science and Technology, Ryukoku University and CREST, Japan Science and Technology Corporation** Mitsubishi Chemical Group Science and Technology Research Center, Inc. and CREST, Japan Science and Technology Corporation*** Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University****
○Yuko Kojima* Kingo Uchida** Norikazu Izumi** Shinichiro Sukata** Shinichiro Nakamura*** Masahiro Irie****
Diarylethene derivatives are promising artificial photoresponsive molecules that show reversible transformation between open- and closed-ring isomers with different absorption spectra. Recently we found photoinduced wettability changes based on the morphology changes of a photochromic diarylethene crystal and thin film.
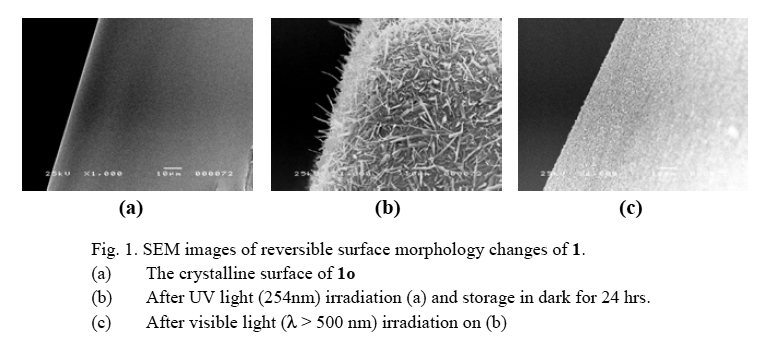
Here we report a new photoinduced surface morphology change, which provides superhydrophobic properties that is obtained by the photoinduced reversible formation of fine fibril structures on coated microcrystalline surfaces. The origin of reversible fibril formation is the photoisomerization of a photochromic diarylethene, 1,2-bis(2-methoxy-5-trimethylsilyl- thien-3-yl)perfluorocyclopentene (1) molecule of thin film. The reversible surface morphology changes of 1 were followed by SEM. (Fig. 1)
X-ray single crystal analysis of open- (1o) and closed-ring (1c) isomers and powder X-ray diffraction measurement were carried out to discover the microfibril composition. The X-ray results indicate that the fibril crystal formed on the film grew up freely from the crystal lattice of the open-ring isomer. We will discuss the reversible microfibril formation mechanism by a phase diagram and structures of 1o and 1c crystals.