Structural Biology Research Center, PF-IMSS-KEK* Department of Molecular Medicine, IMCR, Gunma University** Graduate School of Material Science, NAIST***
○Leonard M G Chavas* Seiji Torii** Hironari Kamikubo*** Masato Kawasaki* Kentaro Ihara* Ryuichi Kato* Mikio Kataoka*** Tetsuro Izumi** Soichi Wakatsuki*
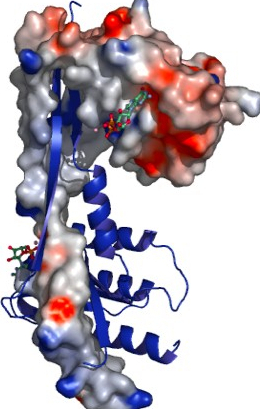
Members of the Rab family of small GTPases regulate membrane traffic within the cell by recruiting their specific effectors in a nucleotide-dependent manner. The Rab27 subfamily consists of Rab27a and Rab27b, which share 70% sequence identity. By interacting with a large set of effector proteins such as melanophilin and granuphilin, both Rab27a/b regulate exocytosis of secretory lysosomes. Here we report the crystallographic structure determination of mouse Rab27b, solved from three distinct lattices in complex with GDP. Surprisingly, Rab27b-GDP exists in an open conformation, stabilized through dimerization by means of domain swapping in the crystals. The high flexibility of the switch domains might be the basis of such unfastening of the enzyme. In contrast, small-angle X-ray scattering measurements demonstrated a monomer form of the enzyme in solution both in its GDP- and GTP-bound states. Potential roles of the dimerization of the inactive Rab27b-GDP along its GTPase cycle will be discussed.
