[1] Koichi Tanaka et al., CryslEngComm., 2004, 6(2), 1-4
Department of Chemistry and Materials Science, Tokyo Institute of Technology* Department of Applied Chemistry, Kansai University, Japan**
○Akiko Sekine* Kumiko Aruga* Hidehiro Uekusa* Katsuya Souno** Koichi Tanaka**
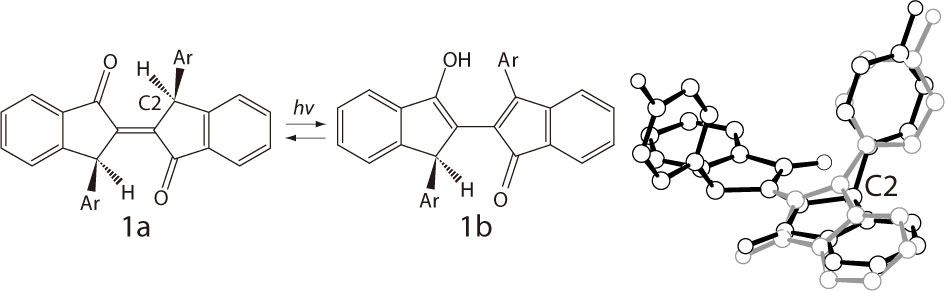
It has been reported that trans-biindenylideneidone derivatives show photochromism in the solid state as evidenced by color change, from yellow(1a) to red(1b), upon irradiation with UV and visible light [1]. However, the crystal structure of the photoproduct (1b) is yet unknown. In this paper, in order to clarify the photochromic reaction mechanism of this compound, we present our experimental results concerning crystal structure determination of 1b. The X-ray analysis of a single crystal of 1a was performed at 173K before the irradiation. Subsequently, the crystal was irradiated with UV light at room temperature and the X-ray diffraction data was collected under the UV light at 173K. The X-ray analysis result shows that both the reactant (1a) and the photoproduct (1b) (11.1%) were located as a disordered structure in the red colored crystal. For 1b, the indenyl and 3-methylphenyl rings are quasi planar. This is due to the proton transfer from C2 to the nearest carbonyl oxygen which causes the change of hybridization from sp3 to sp2. These results suggest that the photochromism is caused by an intramolecular (Norrish Type II) hydrogen transfer reaction.
[1] Koichi Tanaka et al., CryslEngComm., 2004, 6(2), 1-4
