Reference
[1] Lin, L. J.; Ruangrungri, N.; Cordell, G. A.; Shieh, H. L.; Min, Y.; Pezzuto, J. M., Phytochemistry, 31, (1992), 4329-4331.
Research Centre for Bioorganic Chemistry, Department of Chemistry, Faculty of Science, Chulalongkorn University
○Thapong Teerawatananond Nustha Kitpratuang Nattaya Ngamrojnavanich Wilaiwon Tirawanich Amorn Petsom Nongnuj Muangsin
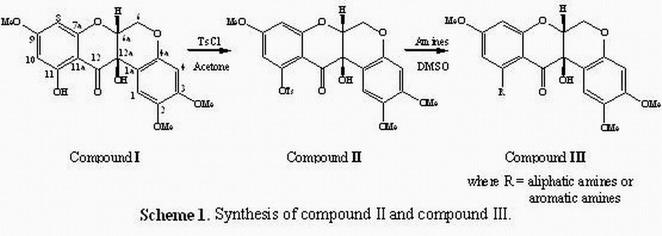
6-Deoxyclitoriacetal is a substance extracted from the dried roots of Stemona collinsae Craib. It has been known to have a cytotoxic activity against various types of human carcinoma possibly due to by its ability to intercalate with DNA as evidenced in vitro assay [1]. In order to enhance its activity, Compound I was derivatised to contain a functional group with more flexible and can be participated hydrogen bonding with DNA. The derivatives of 6-deoxyclitoriacetal were prepared as shown in scheme 1. In this work, we studied the relationship between crystal structures, hydrogen bonding and cytotoxic activity of 6-deoxyclitoriacetal and its derivatives based on spectroscopic and x-ray crystallographic techniques.
Reference
[1] Lin, L. J.; Ruangrungri, N.; Cordell, G. A.; Shieh, H. L.; Min, Y.; Pezzuto, J. M., Phytochemistry, 31, (1992), 4329-4331.
