00362
Crystal structure of a novel family 16 endo-β-1,3-glucanase from Nocardiposis sp. F96
Department of Life Science, Tokyo Institute of Technology* Department of Bioengineering, Tokyo Institute of Technology, Japan**
○Takashi Kumasaka* Guntur Fibriansah* Sumiko Masuda** Satoshi Nakamura**
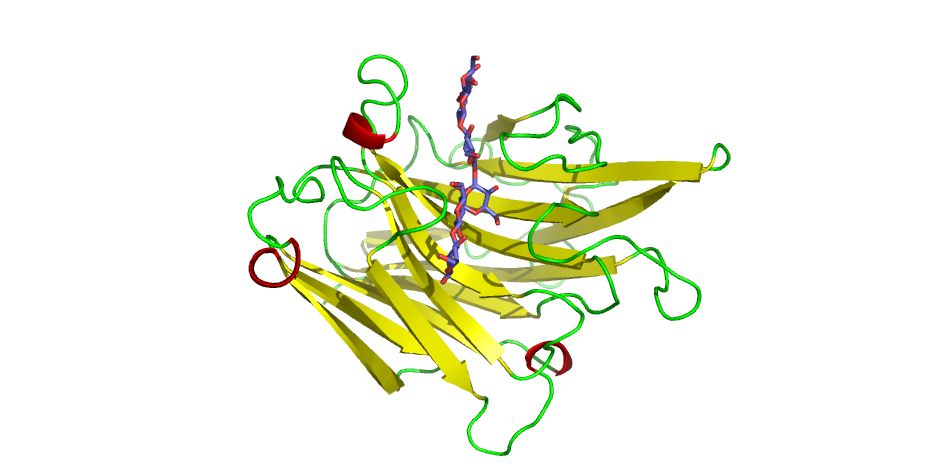
BglF is an endo-β-1,3-glucanase from alkaliphilic Nocardiposis sp. F96 which efficiently hydrolyzes insoluble β-1,3-glucans and shows the highest activity toward a β-1,3-1,4-glucan rather than β-1,3-glucans with optimum temperature of 70°C at pH 6.0. The crystal structure of BglF was determined at 1.3 Å resolution with SAD method of SeMet derivative. The structure shows a jellyroll β-sandwich consisting of seven and eight antiparallel strands whose structure is shared in the glycoside hydrolase (GH) family 16.
The comparison with other GH16 endo-β-1,3-1,4-glucanase structures and the comparative modeling study with two substrate of β-1,3-1,4-glucan tetrasaccharide and laminarihexaose (consisting of β-1,3 linkages mainly, Figure) reveal the 16 residues contributing substrate recognition and suggest that Arg76, Trp118, Trp220 and the some additional loops narrowing the active site might play an important role in the recognition of its substrate. Several possible features including clustered prolines around the active site cleft, ion pairs and packing density that contribute to the thermostability of BglF are elucidated.