[1] A. Kadri et. al., Acta Cryst. D61 (2005) 784.
[2] S. Murakami et. al., Nature 419 (2002) 587.
Graduate School of Engineering, and CREST JST, Osaka Univrsity* Graduate School of Engineering, Osaka University, SOSHO Inc, and CREST JST, Japan** The Institute of Scientific and Industrial Research, Osaka University, SOSHO Inc, and CREST JST, Japan***
○Hiroshi Y. Yoshikawa* Shinya Nakata* Ryota Murai* Tomoya Kitatani* Maki Syou* Hiroaki Adachi** Tsuyoshi Inoue** Hiroyoshi Matsumura** Kazufumi Takano** Satoshi Murakami*** Yusuke Mori** Takatomo Sasaki**
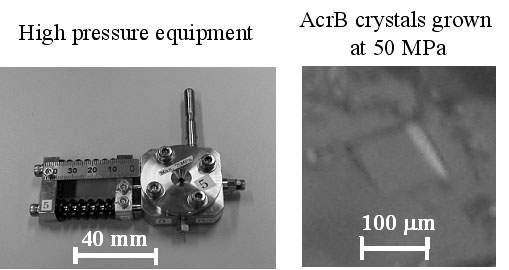
In protein crystallogenesis, pressure is an attractive physical parameter because it can influence structure and/or association of macromolecules in solution. In principle, it affects only the volume of a system and the change in energy is better defined thermodynamically while temperature causes simultaneous changes in the volume and thermal energy of a system. Hence, preliminary pressure studies of model proteins like lysozyme [1] indicated peculiar pressure effects, for example, a reduction of unit cell volume, solubility change, and so on. From the viewpoint, membrane protein seems to be an interesting candidate for a pressure study because the crystals basically have much solvent region inside. Here, we have firstly crystallized membrane protein, AcrB [2], under high pressure. AcrB solubilized with dodecylmaltoside was crystallized using PEG 2000 as a precipitant at 25 °C in specially designed high-pressure equipment. At 50 MPa, we could obtain AcrB crystals with the largest size of 150 mm, which is acceptable size for X-ray diffraction measurement. From the statistical results, the pressure dependence of the crystallization was considered.
[1] A. Kadri et. al., Acta Cryst. D61 (2005) 784.
[2] S. Murakami et. al., Nature 419 (2002) 587.
