 |
 |
|
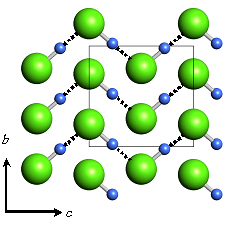
Fig. A The Cmc21 structure of solid HCl of phase III at 18 GPa. |
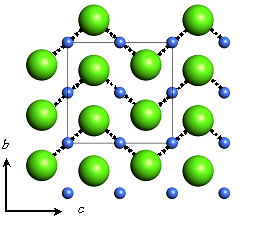
Fig. B The Cmcm structure of solid HCl of phase IV at 55GPa. |
Contact person: Takumi Kikegawa
OFFICE: +81-29-846-5698
BL-18C was constructed in FY 1995 and opened for users from FY 1996. This is a dedicated station for high pressure at high and/or low temperature experiment with a diamond anvil cell (DAC) in order to succeed to the activities of BL-6B1 and B2. DAC is the very compact high-pressure device, which generates pressure in a microscopic sample area by applying a load through the diamonds. Though the pressure is attained more than a 100GPa, the volume which realizes such ultra-high pressure is limited to the area where a diameter and thickness are slight of less than 40mm and 20mm respectively. Diffraction intensity from such a small sample is very weak, so that high intensities are required to obtain reliable intensity data. For this purpose, the the Kirkpatric-Baez type focused optics is adopted. In 1997 a new experimental stage was installed. It is specially designed for the diffraction experiment with DAC. Usually diffracted X-rays are recorded on a flat-type Imaging Plate in order to get precise diffraction data. An X-ray CCD is also available for more quick measurement such as a kinetics study in pressure or temperature induced phase transition.
Light Source: Bending Magnet
Optics:
Monochrometor: Fixed-exit type with two flat Si(111) crystals.
Mirror: Two Pt-coated fused quartz cylindrical mirrors in the Kirkpatric-Baez type arrangement.
Photons at Sample:
Energy: 6-30 keV (Ec=25 keV)
Energy Resolution: ≈2 (DE/E x 10-4)
Photon Flux: ≈1 x 1011/mm2
Beam size: ~40μmV x ≈85μmH (FWHM)
Pinhole collimators: φ25, 40, 60, 80, 100mm.
The IP (two kinds of vertical sizes of 250mm and 400mm with 200mm width) and an X-ray CCD detector C4880 (HAMAMATU Co, Japan; an effective diameter φ160mm) are used to record diffraction patterns. Both detectors are assembled on a slide table with a 500mm-length stroke that is monitored with linear-encoder precisely.
A microscope optical device is installed to adjust the sample position for pressure measurement and X-ray diffraction. The pressure is determined by measuring the pressure-induced wavelength-shift of the ruby fluorescence. In order to excite the fluorescence an Ar laser is applied to the ruby minute particle (around one micron size) that it was put on the sample part of DAC. The microscope is mounted on a pulse motor drive stage, and through the optical fiber ruby fluorescence is led to the monochromator. Laser light is introduced with the optical fiber conversely in the microscope head from the generator. The CCD camera is fitted to the microscope head in order to monitor the sample. A helium gas closed cycle cryostat (LTS-22 RMC) is provided for high-pressure experiment at Low temperature (>10K). Users should bring a miniature DAC with gas membrem (i.e. DXR-GM of DIACELL Products or other DAC with similar size). A off-line pressure measurement optical system is also installed. The system consists of Ar laser, microscope and a monochromator system.
A diffraction pattern on an Imaging-Plat is read-out with BAS2000 and BAS2500 system (Fuji-Film Co.). The pattern (2-dimensional intensity data) can be transformed into a 1-dimensional intensity data by using PIP program (updated by Dr.Fujihisa of NIMC) with network computers connected to BAS system (Ref.).
However there are many beamlines in which high pressure experiment with DAC are carried out in the SR facilities, dedicated beamlines are operating in Europe, US and Japan. One is SAM-From the view point of synchrotron radiation experiment, MAX-III have advantage of synchrotron radiation are similar in terms of energy range and Photon flux.
DAC is well known as a compact and convenient device beyond high pressure experts. Besides those dedicated beamlines, It can be applied most of the beamline in which X-ray diffraction experiment is carried out.
Reference
(1) High-pressure Study of Hydrogen bond Symmetrization on HCl.
HCl is a member of Hydrogen Halide system and is a very popular material for middle and high school students. It is one of the most simple molecule systems containing hydrogen bond. Among the research field of the hydrogen bond system, symmetrization is an advanced topic: when and how a hydrogen bond gains a single well potential with losing its double well potential. Hydrogen bond symmetrization, which has been observed for H2O ice at 60 GPa, can be expected to occur in hydrogen halides system as well. With decreasing temperature HCl gas becomes frozen to a solid state at ambient pressure, which takes three kinds of molecular crystals at different temperature range. They are known as Phase-I, Phase-II and Phase-III and their temperature ranges for existence are 159K>T>120K, 120K>T>98K and 98K>T, respectively. Phase-I and Phase-II have orientationally disordered hydrogen bonds. As for Phase II, a hydrogen atom occupies a symmetrically reversal position. Phase III has a planar zigzag chain of hydrogen bonds.
X-ray powder diffraction experiments on solid HCl was carried out with a diamond anvil cell at room temperature in BL-18C. A pressure-induced transition was observed at 18 GPa and no other phase transition was found up to 61 GPa. The high-pressure phase was identified as Phase-III with space group Cmc21 and pressure effects on its lattice constances and atomic positional parameters were also measured by using Rietvelt analysis.
At 61GPa the interchain Cl-Cl distances in phase III decreases 89% value which is shorter than a doubled van der Waals radii of Chlorine. Continuous color change from brown to black can be explained by this pressure effect on the interchain interaction. The intrachain Cl-H...Cl distance reaches only 5% larger value than a doubled intramolecular distance around 50 GPa. Hydrogen bond symmetrization should take place here judging from this result and a disappearance of molecular vibration in high pressure Raman spectroscopy [1]. Figure shows atomic arrangements of HCl in Phase-III before Hydrogen bond symmetrization at 18GPa [A] and after symmetrization at 55GPa [B]. It is noted that the pressure-induced Hydrogen bond symmetrization means a molecular dissociation in HCl solid simultaneously.
 |
 |
|
Fig. A The Cmc21 structure of solid HCl of phase III at 18 GPa. |
Fig. B The Cmcm structure of solid HCl of phase IV at 55GPa. |
Reference
[1]K.Aoki et al., Physica B 265, 83 (1999)
(2)AMORPHIZATION FROM THE QUENCHED HIGH PRESSURE PHASE IN TETRAHEDRALLY-BONDED MATERIALS
Amorphization from the quenched high pressure phase has been studied for manysemiconductors [1,2]. Results were analysed by using a configuration-coordinate model. As the amorphization depends on the height of potential barrier DU between the two phases of the before- and after-phase transitions, it is interesting to study the path dependence of the phase transition in III-V compounds with different strength and ionicity of bonds.
A diamond anvil cell with a diaphragm was cooled in a cryostat using a He refrigerator. Pressure in the sample was measured by a Ruby fluorescence method with an in-situ pressure measuring system. Pressure can be changed at low temperatures by controlling the He gas pressure in the diaphragm. Diffraction intensity was measured with imaging plate for full Debye rings.
On compression at 300 K, AlSb transforms to the high-pressure phase at about 7 GPa. When the pressure was decreased at 20 K, the high-pressure phase was quenched. With increasing temperature at low pressure, the high pressure phase gradually transforms to an amorphous and microcrystalline phases Path dependences of the phase transitions in AlSb are summarized in Fig.1. Similar experiments were done for GaP. In Fig.2, 1/FWHM of the main peak after decompression is shown as a function of the ionicity. GaSb with a smaller ionicity shows the amorphous patterns, while ZnSe and CdTe with larger ionicities show the patterns of the stable B3 phase. In AlSb and GaP with larger ionicity than that of GaSb, the phase transitions to both the amorphous and microcrystalline phase occur.
From the comparison of the results to the phase transition in other III-V compounds, it is concluded that the amorphization is universal in tetrahedrally-bonded materials. The ionic character in the bonding narrows the region of the amorphization.
Fig. 1 Path dependence of the pressure-induced phase transition in AlSb. nn: the stable zincblende phase; nn: microcrystalline phase with zinc- blende structure; nn : the amorphous phase. |
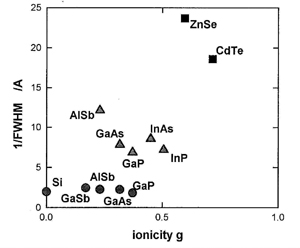
Fig.2. The relation in the tetrahedrally-bonded materials between the ionicity and 1/FWHM of diffraction peak obtained after decompression. (l: amorphous; s: micro crystalline; and n: B3 phase). |
References
[1] M. Imai et al., High Pressure Res., 15, 167 (1996).
[2] K. Tsuji et al., J. Non-Cryst. Solids, 198, 24 (1996); and references therein.
[3] Y. Mori et al., Rev. High Pressure Sci. Technol. 7, 353 (1998).
b) Invited talks, Awards, Symposia
(Inv-1) Hiroshi Fujihisa: ”Structural study of HBr and HCl under Pressure” (Synchrotron, Neutron and Laboratory Source Crystallography at High Pressure: Experiments, theory and related techniques, Nov.14,1988, Argonne National Lab, USA)(Aw-1)Hiroshi Fujihisa: "Structure Physucs on the Mleculer Solids by Powder diffraction Method.”
1998’s An Incentive Award of the Japanese Society of High Pressure Science and Technology.
This MAX-III system always shows a good performance for every users. a For a novel pixel array detector, high throughput electronics has been developed. The new detector and electronics will be in operation in 2001. In order to provide a high flux, an adaptive focusing mirror is needed to be installed in 2001. Also in 2001, a laser system is planned to be installed for pump & probe XAS experiments.
Last modified:
2012-10-01
![]()