 |
Fig. 1 |
Contact person: Takumi Kikegawa
OFFICE: +81-29-846-5698
AR-NE5C was reconstructed in FY1989-90 as a dedicated beamline for high pressure and high temperature X-ray studies. It provides high energy X-rays from a bending magnet of the PF-AR. X-ray experiments are mainly carried out in an Energy-dispersive diffraction method by using white X-rays. However, a double crystal monochromator also available for precise structure analysis in an Angle-dispersive diffraction method. MAX80 is the first high pressure and high temperature experimental system designed for synchrotron radiation use and is the original one of similar systems installed in various SR facilities in the world. It was designed in order to perform various research techniques under high pressure and high temperatures such as a precise determination of lattice constant, dynamical observation of phase transitions and a structural study of new phase and liquid phase utilizing the characteristics of synchrotron radiation.
Light Source: Bending magnet of AR-NE4 (6.5 GeV)
Optics: Majority of experiments use white beam Double-Crystal monochromator
[Si(111)or Ge(111)] is also available in the angle dispersive method.
Photons at Sample:
Energy: 30-100 keV or White
Energy Resolution: ≈5 (ΔE/E x 10-4/) for Si(111)
Beam size: ≈6mmV x ≈60mmH
Press: Maximum loads 500 tons.
Estimated maximum Pressure is 20 GPa (with truncation 3mm anvils)
Anvil system: The DIA-10 type high pressure device
Both sintered diamond and Tungsten-carbide anvils are available.
Diffraction System: 2 axial goniometer with a Ge-SSD for the energy and/or angle
dispersive X-ray diffraction experiment [1].
Detecting and Control System: Main Computer is Pentium-III PC
with MCA(ORTEC-2000C) running Win98 Software
Slit system: Combination of Vertical and Horizontal Slit
with fixed sizes in 50, 100, 200, 300mm.
Similar large volume presses are operating in US, Germany and Japan in view of single-stage anvil system. One is SAM85 installed in X17B1 beamline of NSLS, which is a brother system of MAX-III as designed by Prof. Yagi. The another brother system named MAX80G(*) is installed in F2.1 bending magnet beamline of HASYLAB, designed by the Dr. Shimomura after MAX90. The others are SMAP-I and SMAP-II installed in BL-11XU (Undulator) and BL-14B1(bemding magnet) of Spring-8, respectively. There is no distinction among these systems but it should be pointed out that main experimental techniques have been developed in MAX80. Let them sequence in terms of capacity of high pressure system, MAX80 (500ton), SAM-85 & MAX80-G (250ton), SMAP-I & SMAP-II (150 ton), in the other hand, according to performance of X-ray source, SMAP-II ~MAX80 >MAX80G > SAM85. Considering the up-grade project of AR, MAX80 can get advantage of SMAP-II and the rest.
References
[1] J. Chen, T.Kikegawa, K. Yaoita and O.Shimomura, J. Synchrotron Rad. 4(1997) 21.
(*) As they call their system MAX80 the same name of ours, I call it MAX80G in this article.
(1) Superabundant vacancies in metal-hydrogen alloys
The formation of superabundant vacancies in metal-hydrogen (M-H) alloys is one of the most unique discoveries made by experiments using MAX80. In the course of determination of phase diagrams of M-H systems over wide ranges of hydrogen pressure ( p(H2 ) ≦ 7 Gpa ) and temperature ( T≦1000℃), which has been made possible through our developments of a hydrogen-sealing capsule and internal hydrogen sources, a peculiar contraction of the lattice was found to take place over a long time ( several hours ) [1,2]. Similar lattice contractions have since been observed in a number of fcc and bcc phases of M-H alloys. The observed lattice contraction indicated the formation of an extremely large number of vacancies amounting to as large as ~ 10 at%
Subsequently, a theory was proposed which explained the increase of thermal-equilibrium vacancy concentration as being due to the decrease of the formation energy of a vacancy: The formation energy of a vacancy should be lowered by the sum of binding energies of trapped H atoms [3].
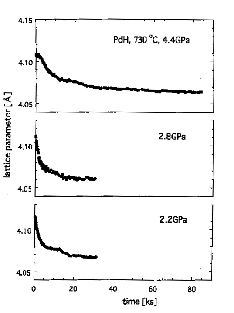
The validity of the theory has since been verified in a number of M-H alloys. As an example, the pressure dependence of the lattice contraction process in Pd-H alloys is shown in Fig.1 [4]. To obtain such high-quality data, the high-precision and long-term stability of the measuring system is essential. From the pressure dependence of the rate and magnitude of contraction, the activation volume of migration and formation of a Vac-H cluster was determined. Similarly, the activation energy of migration and formation was determined from the temperature dependence.
 |
Fig. 1 |
In this way, various features characterizing the superabundant vacancy formation and its consequences have been elucidated. Some of the important consequences include:
(1) The diffusivity of M atoms is enhanced by many orders of magnitude. [5,6].
(2) The most stable structures of M-H alloys are defect structures containing a large number of M-atom vacancies. [7]. Most phase diagrams of M-H systems reported to date are in fact metastable ones because they were obtained under conditions where M-atom vacancies could not be introduced. Still other consequences of superabundant vacancy formation are being expected.
[1] Y.Fukai, N.Okuma, Jpn. J. Appl. Phys. 32 (1993) L1256.
[2] Y.Fukai, N.Okuma, Phys. Rev. Lett. 73 (1994) 1640.
[3] Y.Fukai, Y.Kurokawa, H.Hiraoka, J. Japan Inst. Metals 61 (1997) 663.
[4] Y.Fukai, Y.Ishii, Y.Goto, K.Watanabe, J. Alloys Comp. In press.
[5] K.Watanabe, N.Okuma, Y.Fukai, Y.Sakamoto, Y.Hayashi, Scripta mater. 34 (1996) 551.
[6] E.Hayashi, Y.Kurokawa, Y.Fukai, Phys. Rev. Lett. 80 (1998) 5588.
[7] Y.Fukai, J. Alloys Comp. 231 (1995) 35.
(2) Structure of Liquid Iodine under Pressure
With increasing pressure, crystalline iodine shows a continuous semiconductor-to-metal transition at 16 GPa without molecular dissociation and at 21 GPa a discontinuous transition from a diatomic molecular phase to a monatomic phase, where the intermolecular- and intramolecular-atomic distances jump and both distances become the same[1,2]. On the other hand, liquid iodine shows a semiconductor-to-metal between 3 GPa and 4 GPa. The transition pressure is lower than that for crystalline iodine and a molecular dissociation in the liquid was proposed [3]. We have measured the x-ray diffraction for liquid iodine under high pressure up to 10 GPa by using MAX80 and high energy synchrotron radiation from AR-NE5.
After the correction for the energy distribution of incident x-rays, absorption by the specimen and the pressure-transmitting medium and Compton scattering, the static structure factor S(Q) for the liquid was obtained. Details of experiment and data analysis are described elsewhere[4]. By the Fourier transformation of S(Q), the pair distribution function, g(r), was obtained. In Fig.1, g(r) for liquid iodine at various pressures are shown. The peak at 2.7 A corresponds to the bond length of the diatomic molecules and the second peak at 4.0 A to the intermolecular atomic distance. The peak at 2.7 A remains to 4.8 GPa. With increasing pressure the first peak position of g(r) is nearly constant while the second peak shifts toward shorter distance with d r / d p of 0.056±0.02 A/GPa, which is similar to that in crystalline iodine[1,2].
From these results we conclude that the molecular dissociation of diatomic molecules is not completed at pressures higher than 4 GPa. Pressure of metallization in the liquid state is lower than that in the solid phase at room temperature in iodine similar to sulphur and selenium [3]. Crystalline tellurium is a semiconductor while liquid tellurium is metallic. In liquid tellurium at ambient pressure there exits two kinds of bonds: the long bond and short bond[5,6]. A change in the fraction of the long bond with decreasing temperature was reported for a supercooled liquid tellurium around the metal-to-semiconductor transition temperature [6]. Similar coexistence of two kinds of bonds was also observed in the EXAFS measurements at pressures higher than 4 GPa [7-9]. The semiconductor-to-metal transition in liquid iodine may arise from the coexistence of several kinds of bonds and these cause the large mean square displacement of the nearest neighbour atoms. We propose an another mechanism for the metallization in the liquid state. In liquid iodine, diatomic molecules do not arrange in the same direction as in the crystalline phase. The application of pressure to the liquid iodine causes a decrease in the mean molecular distance. At high pressures the approach of the iodine atoms by thermal rotation should widen the band width of the valence band and narrow the energy gap. The presence of the conduction electrons in the metallic phase also helps the atoms approach. As a result, the metallization in the liquid iodine is expected to occur at pressure lower than that in the crystalline phase.
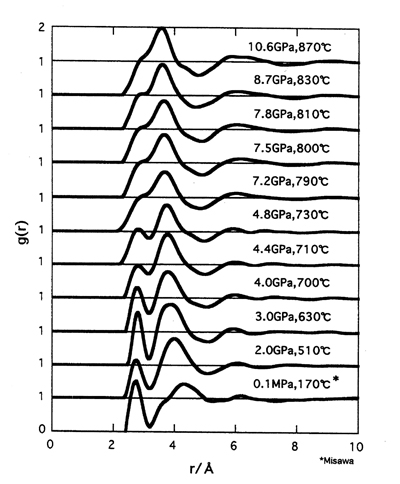
Fig.1 The pair distribution function g(r) for liquid iodine at various pressures. |
References
[1] K. Takemura, S. Minomura, O. Shimomura, Y. Fujii and J. D. Axe, Phys. Rev. B 26, 998 (1982).
[2] Y. Fujii, K. Hase, N. Hamaya, Y. Ohishi, A. Onodera and O. Shimomura, Phys. Rev. Letters, 58, 796 (1987).
[3] V. V. Brazhkin, S. V. Popova, R. N. Voloshin and A. G. Umnov, High Pressure Research 6, 363 (1992).
[4] K. Tsuji, K. Yaoita, M. Imai, O. Shimomura and T. Kikegawa, Rev. Sci. Instrum. 60, 2425 (1989).
[5] K. Tamura, M. Inui, M. Yao, H. Endo, S. Hosokawa, H. Hoshino, Y. Katayama and K. Maruyama, J. Phys.: Condens. Matter 3, 7495 (1991).
[6] T. Tsuzuki, M. Yao and H. Endo, J. Phys. Soc. Jpn. 64, 485 (1995).
[7] Y. Katayama, K. Tsuji, H. Oyanagi and O. Shimomura, J. Non-Cryst. Solids, J. Non-Cryst. Solids 232-234, 93 (1998).
[8] K. Tsuji and Y. Katayama, "The Physics of Complex Liquids", World Science, 1998, p.83.
[9] Y. Katayama, O. Shimomura and K. Tsuji, J. Non-Cryst. Solids 250-252, 537 (1999).
(3)Phase Transitions of Sulfur at High Pressure and High Temperature.
In ordinary elements, the pressure-induced phase transition has been studied well in the pressure range below a few tens GPa. Moreover it has been getting clear about the phase transition over 100GPa by using in-situ x-ray measurement with a diamond anvil cell. As for sulfur, many high-pressure and high-temperature experiments have been carried out from the interest of material science and earth science, but they still lose consistency. It has been assumed that many quasi-stable phases formed by slightly different conditions are found because most of the previous experiments were used quenching method from the high pressure and high temperature condition to the normal condition. In addition, even room temperature x-ray diffraction experiment shows only a complex of broad peaks up to about 20GPa and an amorphous pattern in between 20 to 40GPa[1].
The behavior of sulfur at high pressure and high temperature condition is revealed for the first time in the range of P<16GPa and T< 900℃. High-temperature high-pressure in-situ X-ray diffraction experiment was carried out by using MAX80. Ge-SSD was used for Energy-dispersive method. In this experiment (Fig.1) three kinds of new high pressure phases (b〜d) were found in others of the orthorhombic normal condition phase (a) that is well known as alpha-sulfur with crown-shaped S8 circular molecules. All these new high-pressure phases appear by elevating temperature above 300℃, and they are, as harmony in the usual room temperature high pressure experiments with they weren’t observed yet. High-pressure phases, which appeared at high temperature, could be frozen in the room temperature, although they became unstable with decreasing pressure and couldn't be collected under the normal condition. This result indicates that it is impossible to observe the behavior of sulfur at high pressure and high temperature by quenching method same as previous experiments do.
The possible unit cell of the first high-pressure phase shows a hexagonal unitcell with 9 atoms, that the crystal structure is related to those of hexagonal selenium and tellurium [2]. Selenium atoms form endless zigzag chains along the c-axis in the hexagonal cell. The cell parameters of the first high-pressure phase may be described as a = √3 * aSe, c = cSe and Z = 3 * ZSe, by taking a difference of atomic radius and a compression effect. The fact shows that the high-pressure behavior of sulfur is related to those of selenium and tellurium.
The second high-pressure phase is explained as a hexagonal sulfur, which consists of 18 atoms with S6 circular molecules. The hexagonal sulfur is known as quasi-stable phase at ambient pressure. It is made by re-crystallizing method from the solvent, and has 6% higher density than that of the orthorhombic phase.
It is clear from the ambient pressure phase to the second high-pressure phase, crown-shaped S8 molecule starts to open-ring reaction and put together in another circular molecule S6 caused by this phase transition. As for two other high pressures phases, structure analysis is now proceeding by using DDX (dual-dispersive X-ray diffraction) method [3].
Fig 1. X-ray diffraction patterns of Sulfur. a) The ambient pressure phase, b) the high-pressure phase I, c) the high-pressure phase II, and the high-pressure phase III.
|
Reference
[1] Akahama et.al., Proc.AIRAPT-14, 425 (1994) and references there in.
[2] W. G. Wyckoff, Crystal Structures, vol.1, p.36 (1982).
[3] T.Kikegawa, J.Chen, K.Yaoita and O.Shimomura, Rev. Sci.Instrum.66(2), 1335 (1995)
The paper concerning the first topic was given the 1997’s best paper award from Japan Institute of Metals.
Y.Fukai, Y.Kurokawa, H.Hiraoka, J. Japan Inst. Metals 61 (1997) 663.
In this summer shutdown, all pulse-motor control system and X-ray detecting system will replaced by new systems like MAX-III including data analyzing software. This MAX80 system was installed in 1983. From the beginning many improvements and developments have been achieved to contribute a good performance and an extension of research field to every users. It will be the next step for high pressure science to bring MAX80 into AR-NE1 station, in which high intense synchrotron radiation from the MPW is available.
Last modified:
2012-10-01
![]()